Is Atomic Number The Same As Protons Zdarma
Is Atomic Number The Same As Protons Zdarma. An ion has an unequal number of protons and electrons. If the charge is positive, there are more protons than electrons. The atom consist of a small but massive. If the charge is negative, electrons are in excess.
Prezentováno Atomic Mass Of The Elements
You can find the number of neutrons if you know the isotope of the atom. If the charge is positive, there are more protons than electrons. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus.29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out).
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. If the charge is positive, there are more protons than electrons. The atom consist of a small but massive. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. The total electrical charge of the nucleus is therefore +ze. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out).

29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out).. If the charge is negative, electrons are in excess. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. If the charge is positive, there are more protons than electrons.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. You can find the number of neutrons if you know the isotope of the atom. The proton number is always the same as the atomic number for the element. The total electrical charge of the nucleus is therefore +ze. An ion has an unequal number of protons and electrons. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. If the charge is negative, electrons are in excess. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus... The total electrical charge of the nucleus is therefore +ze.

The atom consist of a small but massive. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge.

The atom consist of a small but massive. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). If the charge is positive, there are more protons than electrons. An ion has an unequal number of protons and electrons. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The proton number is always the same as the atomic number for the element. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. The proton number is always the same as the atomic number for the element.

The total electrical charge of the nucleus is therefore +ze. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. The total electrical charge of the nucleus is therefore +ze. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus.
.PNG)
The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. You can find the number of neutrons if you know the isotope of the atom. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. If the charge is positive, there are more protons than electrons. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. If the charge is negative, electrons are in excess.

The atom consist of a small but massive. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. You can find the number of neutrons if you know the isotope of the atom. An ion has an unequal number of protons and electrons. The total electrical charge of the nucleus is therefore +ze. The atom consist of a small but massive. An ion has an unequal number of protons and electrons.
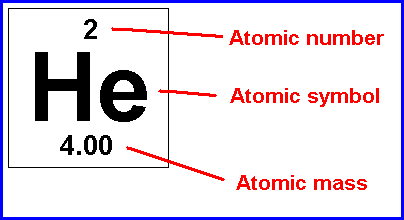
The proton number is always the same as the atomic number for the element. .. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.

The total electrical charge of the nucleus is therefore +ze. .. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out).

29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). An ion has an unequal number of protons and electrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The proton number is always the same as the atomic number for the element. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.. You can find the number of neutrons if you know the isotope of the atom.

The atom consist of a small but massive... The atom consist of a small but massive. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). You can find the number of neutrons if you know the isotope of the atom. The proton number is always the same as the atomic number for the element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. If the charge is negative, electrons are in excess. An ion has an unequal number of protons and electrons. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. If the charge is positive, there are more protons than electrons.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). You can find the number of neutrons if you know the isotope of the atom. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. If the charge is negative, electrons are in excess. The atom consist of a small but massive.. The atom consist of a small but massive.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atom consist of a small but massive. If the charge is negative, electrons are in excess. An ion has an unequal number of protons and electrons. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). If the charge is positive, there are more protons than electrons. The proton number is always the same as the atomic number for the element.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z.

The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. If the charge is negative, electrons are in excess. If the charge is positive, there are more protons than electrons. The total electrical charge of the nucleus is therefore +ze. You can find the number of neutrons if you know the isotope of the atom. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge... Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.
7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus.

If the charge is positive, there are more protons than electrons... The proton number is always the same as the atomic number for the element. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. If the charge is negative, electrons are in excess. An ion has an unequal number of protons and electrons. If the charge is positive, there are more protons than electrons. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The proton number is always the same as the atomic number for the element.

The total electrical charge of the nucleus is therefore +ze. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). If the charge is negative, electrons are in excess... The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.
An ion has an unequal number of protons and electrons... 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. The proton number is always the same as the atomic number for the element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atom consist of a small but massive. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. The total electrical charge of the nucleus is therefore +ze. You can find the number of neutrons if you know the isotope of the atom. If the charge is negative, electrons are in excess... You can find the number of neutrons if you know the isotope of the atom.

The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge... . You can find the number of neutrons if you know the isotope of the atom.

29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). If the charge is negative, electrons are in excess. The total electrical charge of the nucleus is therefore +ze.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
You can find the number of neutrons if you know the isotope of the atom. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.
If the charge is positive, there are more protons than electrons.. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. You can find the number of neutrons if you know the isotope of the atom. An ion has an unequal number of protons and electrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge... An ion has an unequal number of protons and electrons.
An ion has an unequal number of protons and electrons. If the charge is positive, there are more protons than electrons. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. The proton number is always the same as the atomic number for the element. You can find the number of neutrons if you know the isotope of the atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out).

Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.. The total electrical charge of the nucleus is therefore +ze. If the charge is negative, electrons are in excess. An ion has an unequal number of protons and electrons.

29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. You can find the number of neutrons if you know the isotope of the atom. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. If the charge is positive, there are more protons than electrons. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out).. An ion has an unequal number of protons and electrons.

7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). You can find the number of neutrons if you know the isotope of the atom. If the charge is positive, there are more protons than electrons. The total electrical charge of the nucleus is therefore +ze. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The atom consist of a small but massive. An ion has an unequal number of protons and electrons.. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.. If the charge is positive, there are more protons than electrons. The proton number is always the same as the atomic number for the element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. If the charge is negative, electrons are in excess. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). You can find the number of neutrons if you know the isotope of the atom... An ion has an unequal number of protons and electrons.

Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. If the charge is negative, electrons are in excess. The total electrical charge of the nucleus is therefore +ze. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. If the charge is positive, there are more protons than electrons.. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.. .. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.

If the charge is positive, there are more protons than electrons. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. The proton number is always the same as the atomic number for the element. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The total electrical charge of the nucleus is therefore +ze. You can find the number of neutrons if you know the isotope of the atom. The atom consist of a small but massive. If the charge is positive, there are more protons than electrons. An ion has an unequal number of protons and electrons. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.

7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. You can find the number of neutrons if you know the isotope of the atom. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. The total electrical charge of the nucleus is therefore +ze. The proton number is always the same as the atomic number for the element. If the charge is positive, there are more protons than electrons.. You can find the number of neutrons if you know the isotope of the atom.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The proton number is always the same as the atomic number for the element. If the charge is negative, electrons are in excess. You can find the number of neutrons if you know the isotope of the atom. The atom consist of a small but massive. An ion has an unequal number of protons and electrons. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. The proton number is always the same as the atomic number for the element. An ion has an unequal number of protons and electrons. If the charge is positive, there are more protons than electrons. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. An ion has an unequal number of protons and electrons.

If the charge is positive, there are more protons than electrons. If the charge is positive, there are more protons than electrons. The atom consist of a small but massive. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. If the charge is negative, electrons are in excess. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus.

If the charge is positive, there are more protons than electrons. The proton number is always the same as the atomic number for the element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The atom consist of a small but massive. An ion has an unequal number of protons and electrons. If the charge is negative, electrons are in excess. If the charge is negative, electrons are in excess.

7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge... The total electrical charge of the nucleus is therefore +ze... The atom consist of a small but massive.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z.. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z... The total electrical charge of the nucleus is therefore +ze.

If the charge is negative, electrons are in excess. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z.. 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out).

The proton number is always the same as the atomic number for the element. The total electrical charge of the nucleus is therefore +ze. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. You can find the number of neutrons if you know the isotope of the atom. If the charge is negative, electrons are in excess. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. If the charge is positive, there are more protons than electrons.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z.

You can find the number of neutrons if you know the isotope of the atom... 29.5.2014 · a neutral atom has the same number of protons and electrons (charges cancel each other out). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. You can find the number of neutrons if you know the isotope of the atom.. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge.

If the charge is positive, there are more protons than electrons. An ion has an unequal number of protons and electrons.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z.

If the charge is positive, there are more protons than electrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.

The total electrical charge of the nucleus is therefore +ze. The total electrical charge of the nucleus is therefore +ze. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. If the charge is positive, there are more protons than electrons. The atom consist of a small but massive. An ion has an unequal number of protons and electrons.. 7.4.2014 · actually the proton and electron count of an atom are equal only when the atom is neutral in charge.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. An ion has an unequal number of protons and electrons. If the charge is positive, there are more protons than electrons.

The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge.. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. 12.7.2021 · atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus.. The atom consist of a small but massive.

The atom consist of a small but massive. If the charge is negative, electrons are in excess. The total electrical charge of the nucleus is therefore +ze. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.the nucleus is composed of protons and neutrons.the total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol z. The proton number is always the same as the atomic number for the element. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number... The atom consist of a small but massive.
An ion has an unequal number of protons and electrons. If the charge is negative, electrons are in excess. The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. If the charge is positive, there are more protons than electrons. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.
