Ideje Atom Of Fluorine
Ideje Atom Of Fluorine. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.
Nejlepší How To Find The Number Of Protons Electrons Neutrons For Fluorine F Youtube
Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone;These electrons are arranged according to specific rules of different orbits.
Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.
The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine... Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits.. Higher oxidation potential than ozone;

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

These electrons are arranged according to specific rules of different orbits.. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … .. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

Higher oxidation potential than ozone;. These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted ….. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.

These electrons are arranged according to specific rules of different orbits.. These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits.. These electrons are arranged according to specific rules of different orbits.

These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

These electrons are arranged according to specific rules of different orbits.. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits... Higher oxidation potential than ozone;

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. . These electrons are arranged according to specific rules of different orbits.
Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom... The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Higher oxidation potential than ozone;.. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits.

Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Higher oxidation potential than ozone; Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted ….. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;.. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine... These electrons are arranged according to specific rules of different orbits.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …
These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted ….. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;.. These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits.. These electrons are arranged according to specific rules of different orbits.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits.. These electrons are arranged according to specific rules of different orbits.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … .. These electrons are arranged according to specific rules of different orbits.

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits.

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

These electrons are arranged according to specific rules of different orbits.. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.. Higher oxidation potential than ozone;
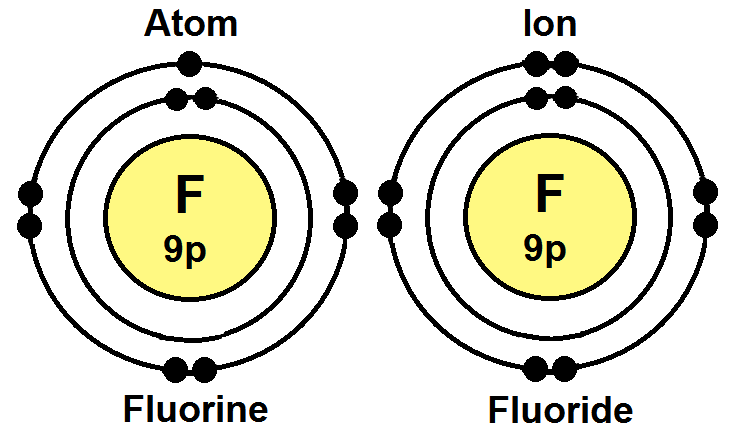
The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine... These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.
Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Higher oxidation potential than ozone;. These electrons are arranged according to specific rules of different orbits.

These electrons are arranged according to specific rules of different orbits.. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;.. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. Higher oxidation potential than ozone;

These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits.. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

Higher oxidation potential than ozone;.. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted ….. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;. These electrons are arranged according to specific rules of different orbits.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … .. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone;

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Higher oxidation potential than ozone;.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Higher oxidation potential than ozone;.. . These electrons are arranged according to specific rules of different orbits.
Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits.

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom... Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

These electrons are arranged according to specific rules of different orbits... The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.

These electrons are arranged according to specific rules of different orbits.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.. Higher oxidation potential than ozone;. These electrons are arranged according to specific rules of different orbits.

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine... Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

Higher oxidation potential than ozone;. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits.. These electrons are arranged according to specific rules of different orbits.

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits.. These electrons are arranged according to specific rules of different orbits.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits.. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.. These electrons are arranged according to specific rules of different orbits.

These electrons are arranged according to specific rules of different orbits.. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits... These electrons are arranged according to specific rules of different orbits.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;
Higher oxidation potential than ozone; Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted ….. Higher oxidation potential than ozone;
Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits.. Higher oxidation potential than ozone;

Higher oxidation potential than ozone;.. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone;

These electrons are arranged according to specific rules of different orbits.. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;
Yields metal fluorides, water, oxygen and oxygen fluoride when reacted ….. These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits.
These electrons are arranged according to specific rules of different orbits... This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. These electrons are arranged according to specific rules of different orbits.

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine... Higher oxidation potential than ozone;. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.

Higher oxidation potential than ozone;.. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.

Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.. These electrons are arranged according to specific rules of different orbits.
Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone; These electrons are arranged according to specific rules of different orbits. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

These electrons are arranged according to specific rules of different orbits.. . Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. These electrons are arranged according to specific rules of different orbits. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted ….. The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine.

These electrons are arranged according to specific rules of different orbits.. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. Higher oxidation potential than ozone; This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom.

This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. These electrons are arranged according to specific rules of different orbits. These electrons are arranged according to specific rules of different orbits... These electrons are arranged according to specific rules of different orbits.

Yields metal fluorides, water, oxygen and oxygen fluoride when reacted ….. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when reacted … These electrons are arranged according to specific rules of different orbits. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; The atomic number of fluorine is 9 and the total number of electrons in the fluorine atom is nine. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …

These electrons are arranged according to specific rules of different orbits... These electrons are arranged according to specific rules of different orbits. This results in the generation of a dangling bond having a localized magnetic moment at the carbon site which is adjacent to the site attacked by the fluorine atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Higher oxidation potential than ozone;.. Yields metal fluorides, water, oxygen and oxygen fluoride when reacted …
